University of Oxford
Coronavirus vaccine breakthrough raises hopes of rapid global rollout
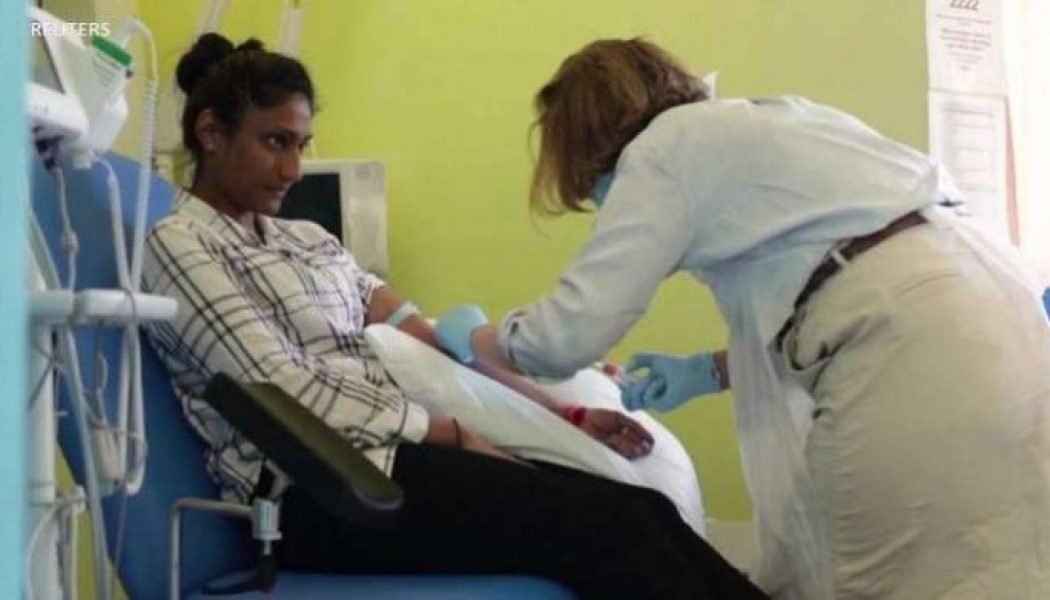
A coronavirus vaccine developed by Britain’s University of Oxford and the pharmaceutical firm AstraZeneca has shown successful results in early trials. If it is approved by regulators, the vaccine appears suitable for a fast rollout around the globe. Early analysis of trials involving 20,000 volunteers in Britain and Brazil show the vaccine is at least 62% effective after two doses. In volunteers given a different dosing regimen — a half dose, followed by a full dose — that figure rose to 90%. The average efficacy of the two dosing methods is 70%. None of those given the vaccine developed severe COVID-19 illness. Andrew Pollard, director of the Oxford Vaccine Group, said the recent successful trials of three different vaccines by Oxford-AstraZeneca, Pfizer-BioNTech and Moderna, represent a...
Two coronavirus vaccines proven safe for human – research
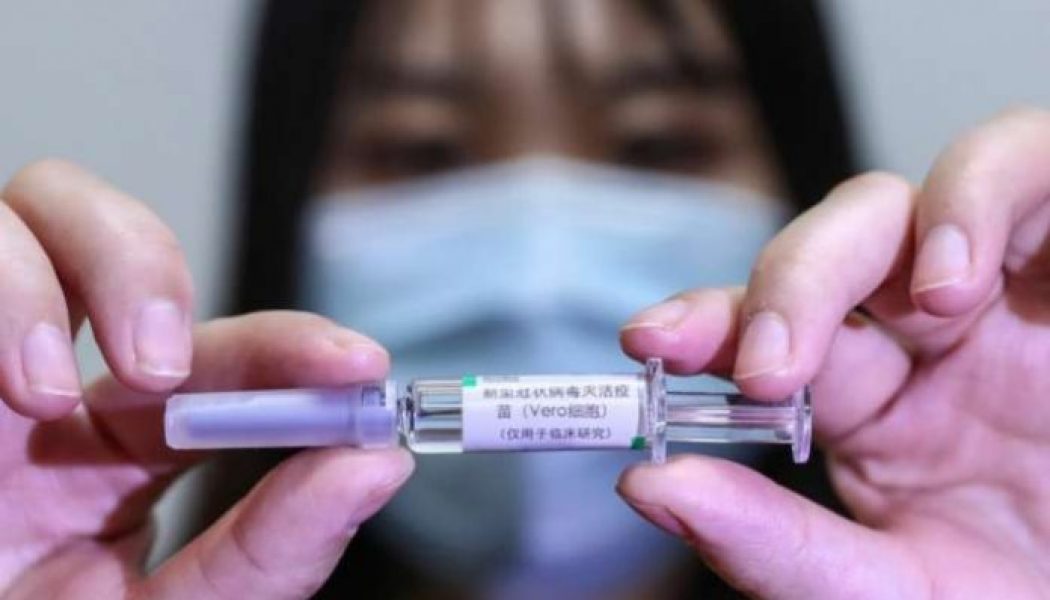
Two coronavirus vaccine have proven safe for humans and produced strong immune reactions among patients involved in separate clinical trials, doctors said Monday. The first trial among more than 1,000 adults in Britain found that the vaccine-induced “strong antibody and T cell immune responses” against the novel coronavirus. A separate trial in China involving more than 500 people showed most had developed widespread antibody immune response. The studies, published in The Lancet medical journal, constitute a major step on the road towards a COVID-19 vaccine that is effective and safe for widespread use. The authors of the studies said that they encountered a few adverse side effects from the vaccine candidates. However, they cautioned that more research was needed, particularly among older...