European Medicines Agency
France: Coronavirus vaccine will be free for all
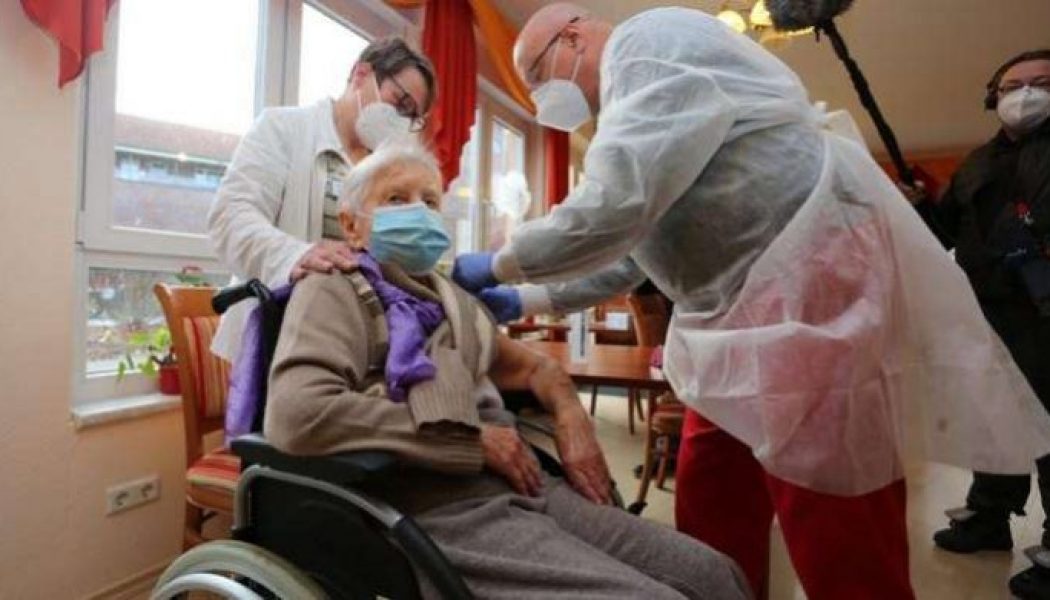
France will ensure free COVID-19 vaccinations for all who are in its social security system and has earmarked 1.5 billion euros of next year’s social security budget to cover the cost, Prime Minister Jean Castex said on Thursday. Castex said the vaccination campaign would begin in a matter of weeks, pending regulatory approval by the European Medicines Agency. The inoculation programme would be staggered over three categories of people, he said, commencing with the most vulnerable in nursing homes. “The vaccination will be free for all,” Castex told a press conference. France has ordered some 200 million doses from different pharmaceutical companies developing vaccines, Castex said, enough to inoculate 100 million people – more than France’s population. The COVID-19 vaccination will be vol...