AstraZeneca
Coronavirus vaccine breakthrough raises hopes of rapid global rollout
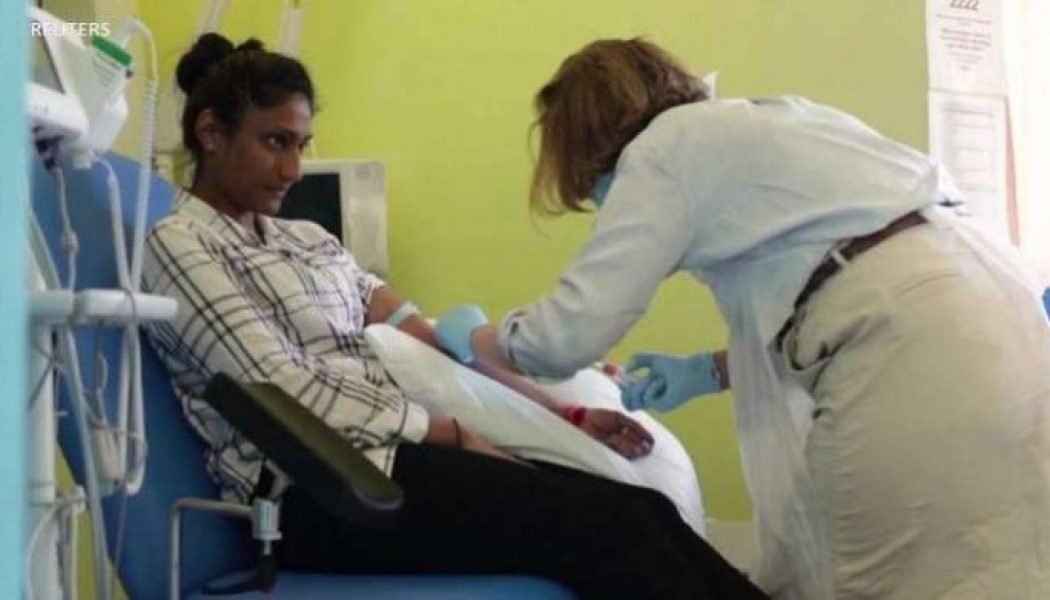
A coronavirus vaccine developed by Britain’s University of Oxford and the pharmaceutical firm AstraZeneca has shown successful results in early trials. If it is approved by regulators, the vaccine appears suitable for a fast rollout around the globe. Early analysis of trials involving 20,000 volunteers in Britain and Brazil show the vaccine is at least 62% effective after two doses. In volunteers given a different dosing regimen — a half dose, followed by a full dose — that figure rose to 90%. The average efficacy of the two dosing methods is 70%. None of those given the vaccine developed severe COVID-19 illness. Andrew Pollard, director of the Oxford Vaccine Group, said the recent successful trials of three different vaccines by Oxford-AstraZeneca, Pfizer-BioNTech and Moderna, represent a...
Europe to pay less than US for Pfizer COVID-19 vaccine
The European Union has struck a deal to initially pay less for Pfizer’s COVID-19 vaccine candidate than the United States, an EU official told Reuters News Agency as the bloc announced on Wednesday it had secured an agreement for up to 300 million doses. The experimental drug, developed in conjunction with Germany’s BioNTech, is the frontrunner in a global race to produce a vaccine, with interim data released on Monday showing it was more than 90 percent effective at protecting people from COVID-19 in a large-scale clinical trial. Under the EU deal, 27 European countries could buy 200 million doses, and have an option to buy another 100 million. The bloc will pay less than $19.50 per jab, a senior EU official involved in talks with vaccine makers told Reuters, adding that partly reflected ...