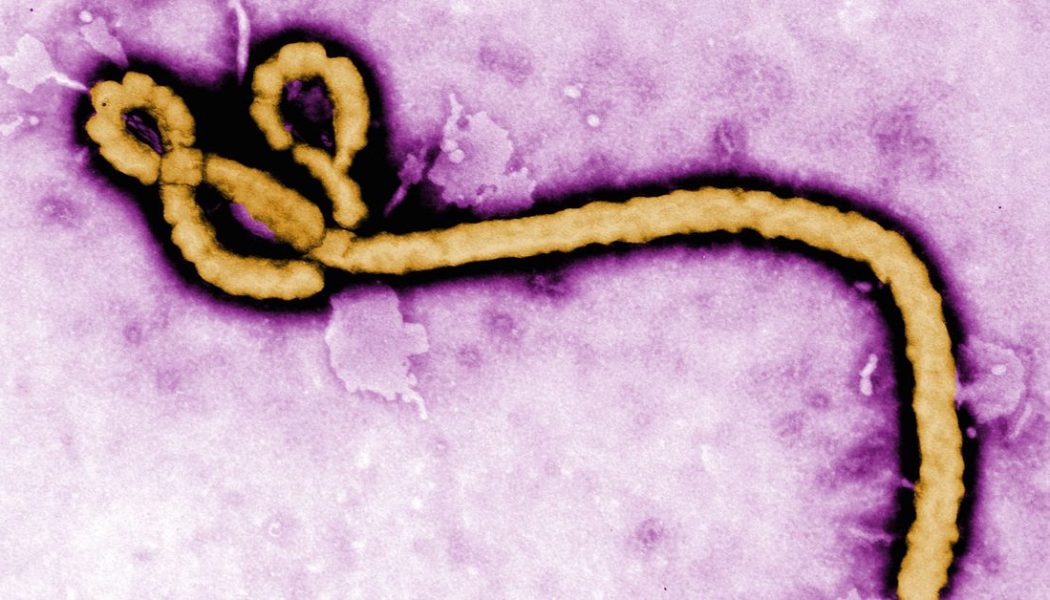
The Food and Drug Administration approved a drug tested during the 2018 Ebola epidemic as a treatment for the disease. It’s the first therapy approved by the agency for Ebola, and shows that research done during an emergency can find effective drugs.
“Today’s approval highlights the importance of international collaboration in the fight against Ebola virus,” said John Farley, director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research.
The drug, called Inmazeb, is a mixture of three antibodies that block the Ebola virus. It was developed by Regeneron, which is also testing an antibody treatment for COVID-19. In clinical trials, patients who took Inmazeb were far less likely to die from the disease.
Inmazeb was tested in a clinical trial that ran in 2018 and 2019 during an outbreak of Ebola in the Democratic Republic of the Congo. The trial, called the PALM trial, compared four drugs developed to treat Ebola, and two — this drug and a different antibody cocktail — proved to be the most effective.
The PALM trial was the first major attempt to run a rigorous clinical trial during an ongoing disease outbreak. Before it started, the World Health Organization was worried about the downsides of conducting a trial during a emergency, because it could take resources away from caring for sick patients. This trial and other studies done during Ebola outbreaks over the past decade showed that scientific research during an epidemic was possible.
Researchers are now applying those lessons during the COVID-19 pandemic. One of the US-based trials for the antiviral remdesivir, which the FDA authorized for emergency use, was modeled after the PALM trial, for example.
“What we learned from Ebola is definitely something that is helping us to be even better during this outbreak,” Andre Kalil, a professor of internal medicine at the University of Nebraska Medical Center, told The Verge in February.