Abstract
We investigated the complex relations of socioeconomic status (SES) and healthy lifestyles with cognitive functions among older adults in 1313 participants, aged 60 years and older, from the National Health and Nutrition Examination Survey 2011–2014. Cognitive function was measured using an average of the standardized z-scores of the Consortium to Establish a Registry for Alzheimer’s Disease Word Learning and delayed recall tests, the Animal Fluency Test, and the Digit Symbol Substitution Test. Latent class analysis of family income, education, occupation, health insurance, and food security was used to define composite SES (low, medium, high). A healthy lifestyle score was calculated based on smoking, alcohol consumption, physical activity, and the Healthy-Eating-Index-2015. In survey-weighted multivariable linear regressions, participants with 3 or 4 healthy behaviors had 0.07 (95% CI 0.005, 0.14) standard deviation higher composite cognitive z-score, relative to those with one or no healthy behavior. Participants with high SES had 0.37 (95% CI 0.29, 0.46) standard deviation higher composite cognitive z-score than those with low SES. No statistically significant interaction was observed between healthy lifestyle score and SES. Our findings suggested that higher healthy lifestyle scores and higher SES were associated with better cognitive function among older adults in the United States.
Introduction
Dementia currently affects approximately 50 million people worldwide, and this number is expected to rise to 152 million by 20501. Maintaining cognitive function is crucial for promoting the health of the aging population. Individuals with low socioeconomic status (SES) are more likely to experience impaired cognitive function2,3,4,5,6. SES has also been associated with functional and structural neural differences in wide range of cortical areas7. Despite increasing life expectancy, there are widening SES inequalities in health8. At this time, treatments for dementia are inadequate9. Identifying susceptible populations and modifiable risk factors for cognitive decline are priorities for public health intervention10. Comprehensive strategies are warranted to identify vulnerable groups who may benefit most from preventive interventions to reduce cognitive declines and socioeconomic disparities.
Most emphasis is now directed towards individual-level interventions on potentially modifiable lifestyle factors11. Diets rich in fruit and vegetables, abstinence from smoking, and regular exercise are associated with better cognitive performance and lower risk of dementia12,13,14. Despite extensive investigations, clear understanding of the roles of healthy lifestyles and SES in cognitive health is still lacking15. Few attempts have been made to investigate associations between combined lifestyle factors and cognitive function. For studies focusing on SES, most previous studies used single socioeconomic variables (e.g., education, occupation, income) to represent SES16,17. SES is a complex, multifactorial construct, and composite SES measurements reflecting multiple socioeconomic factors are needed to characterize18,19. Furthermore, evidence suggests that healthy lifestyles could play a role in health outcomes patterned by SES, particularly cardiometabolic disease and mortality15; yet, it is unknown whether healthy lifestyles could alleviate socioeconomic inequalities in cognitive health.
We used data from the National Health and Nutrition Examination Survey (NHANES) to examine the complex relationships of SES and lifestyle factors with cognitive function in older adults. We defined individual-level SES with a composite of education, occupation, income-to-poverty ratio, health insurance, and food security. A composite healthy lifestyle score was constructed based on health behaviors including smoking status, alcohol consumption, physical activity, and diet. We hypothesized that higher composite SES and healthy lifestyle scores were associated with better cognitive function in a cross-sectional sample of U.S. older adults.
Results
Univariate analyses: participant characteristics and cognitive function by SES and healthy lifestyle score
The analytic sample had a mean age of 69.3 years. Latent class analysis (LCA) was used to classify SES based on multiple factors (Table S2). The high SES class was characterized by income-to-poverty ratio ≥ 4, white collar occupations, college education or higher, health insurance, and full food security. The medium SES class was characterized by income-to-poverty ratio > 1 and < 4, blue collar occupation, and food security. The low SES class was characterized by income-to-poverty ratio < 4, blue collar occupations, less than high school education, government insurance or uninsured, and food insecurity. Within the sample, 581 were of high SES, 351 were of medium SES, and 381 were of low SES. Low SES participants tended to be women, non-White people, not married, born outside of the United States, have less alcohol consumption, and less healthy diets (Table 1). For healthy lifestyles, 341 study participants had 0 or 1 healthy behavior, 512 had 2 healthy behaviors, and 460 had 3 or 4 healthy behaviors (Table 2). Participants with less healthy behaviors were more likely to be non-White people, born in the United States, and have blue collar occupations.
Table 3 presents the distributions of the cognitive composite z-score and its components. Participants with higher SES, on average, had higher test scores of Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) delayed recall, Animal Fluency, Digit Symbol Substitution Test (DSST), as well as the composite z-score, compared to participants with lower SES. The mean (SE) test scores were 6.7 (0.1) for CERAD delayed recall, 19.8 (0.3) for Animal Fluency, 58.8 (0.7) for DSST, and 0.41 (0.02) for the composite z-score among participants with high SES. In contrast, the mean (SE) test scores were 5.7 (0.2) for CERAD delayed recall, 16.2 (0.4) for Animal Fluency, 41.8 (1.5) for DSST, and -0.07 (0.04) for the composite z-score among participants with low SES.
Primary analysis: adjusted associations between composite exposure measures and cognitive score
To assess the associations between cumulative SES and lifestyle factors, we tested the adjusted relationships of healthy lifestyle score and composite SES with composite cognitive z-score (Table 4). Participants with 3 or 4 healthy behaviors, on average, had 0.07 (95% CI 0.005, 0.14) standard deviation higher composite cognitive z-scores than those with one or less healthy behavior. Participants with high SES, on average, had 0.37 (95% CI 0.29, 0.46) standard deviation higher composite cognitive z-scores than those with low SES.
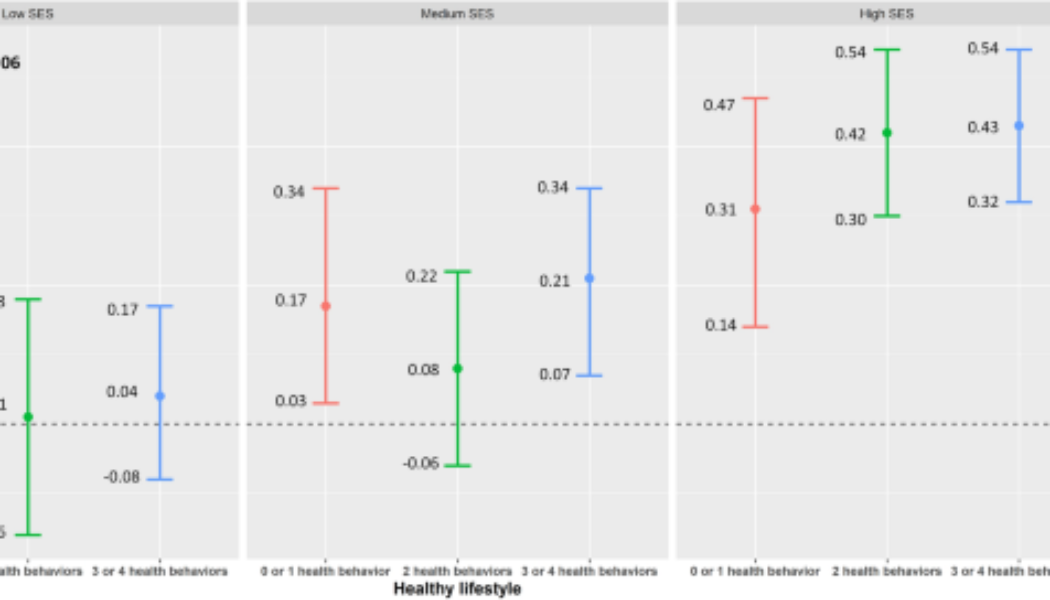
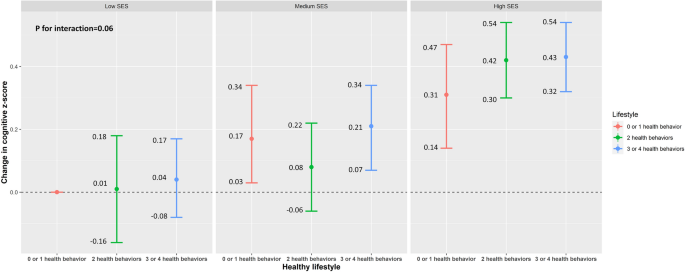
Figure 1 presents the joint associations of lifestyle and SES with composite cognitive z-score, according to the combination of the nine categories of healthy lifestyle score and composite SES. The interaction between the healthy lifestyle score and composite SES was not statistically significant (P for interaction = 0.06). Participants with high SES had better cognitive function compared to those with low SES, independent of the healthy lifestyle score.
Survey-weighted changes in cognition z-score with an additional interaction term between socioeconomic status (SES) and healthy lifestyle from the U.S. National Health and Nutrition Examination Survey 2011–2014. Model was adjusted for age, sex, race/ethnicity, marital status, and birthplace. P for interaction between SES and healthy lifestyle was 0.06.
Secondary analysis: adjusted associations between individual exposure measures and cognitive score
We tested the associations between individual healthy lifestyle behaviors (mutually adjusted) and cognition score, adjusting for age, sex, race/ethnicity, marital status, and birthplace (Model 1, Table 5). On average, participants with low or moderate alcohol consumption had 0.11 (95% CI -0.21, -0.02) standard deviation lower cognitive score compared to those with high alcohol consumption. Those with healthy physical activity levels had 0.08 (95% CI 0.03, 0.14) standard deviation higher cognitive scores compared to those with unhealthy physical activity. Those with healthy eating had 0.08 (95% CI 0.02, 0.15) standard deviation higher composite cognitive z-scores, compared to those with unhealthy eating habits.
We tested the similarly adjusted associations between SES components and cognition score (Model 2, Table 5). We observed participants in the highest categories of family income (income-to-poverty ratio ≥ 4) had 0.15 (95% CI 0.03, 0.27) standard deviation higher composite cognitive scores, compared to those in the lowest categories (income-to-poverty ratio ≤ 1 for family income). Participants in the highest category of education (college and above) had 0.26 (95% CI 0.16, 0.37) standard deviation higher composite cognitive z-scores, compared to those in the less than high school education category. Participants with food security had 0.13 (95% CI 0.06, 0.20) standard deviation higher composite cognitive z-scores than those with food insecurity. Participants who had white collar occupations had 0.16 (95% CI 0.08, 0.24) standard deviation higher composite cognitive z-scores than those who had blue collar occupations.
We then included both lifestyle and SES components in similarly adjusted models of cognitive score (Model 3, Table 5). Similar associations were observed between all of SES components and cognitive z-score to the previous models without lifestyle component adjustment. However, after adjusting for SES components, physical activity was the only lifestyle factor still associated with cognitive z-score.
Sensitivity results
In sensitivity analyses of individual cognitive assessments, higher SES was associated with higher cognition measured with the CERAD immediate learning, Animal Fluency, and DSST (Table S3). When examining the associations using the weighted healthy lifestyle score, a similar positive association was observed (Table S4). Additional adjustment for body mass index (BMI) did not alter the associations (Table S5). In a subpopulation after excluding 283 participants with cardiovascular disease or stroke, similar associations of healthy lifestyle score and composite SES with composite cognitive z-score were observed (Tables S6 and S7). Similar associations were observed in the pooled analysis of 20 imputed datasets (Table S8).
Discussion
This study examined the associations of lifestyle and socioeconomic factors with cognitive function in a representative sample of adults aged 60 years and older in the United States. We summarized smoking status, alcohol consumption, physical activity, and healthy diet in a composite healthy lifestyle score and education, family income, occupation, health insurance, and food security into a composite SES. Both higher healthy lifestyle scores and higher SES scores were independently associated with better cognitive function. These associations persisted after controlling for age, sex, race, marital status, and birthplace. The magnitude of the SES effect was considerably greater than the magnitude of healthy lifestyle factors. In individual component analyses, participants with higher physical activity, higher education, higher family income, better health insurance, food security, and white-collar occupations had better cognitive function performance. This study is the first to investigate the complex relationships between SES, healthy lifestyles, and cognition.
Given population ageing and lack of therapies to halt the progression to dementia, identification of vulnerable populations and modifiable risk factors for age-related impaired cognitive function is of substantial public health importance. Lifestyle factors are potentially modifiable factors. The associations of various individual lifestyle factors such as healthy diet20, physical activity21, and smoking22 with cognitive function and dementia were described previously, which is consistent with our study results. Additionally, we evaluated the combinations of multiple lifestyle factors given that those factors are not independent of each other and the complex and multifactorial etiology of cognitive impairment and dementia. Three large trials examined the effects of multiple lifestyle interventions on cognitive function—the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), the French Multidomain Alzheimer Preventive Trial (MAPT)23 and the Dutch Prevention of Dementia by Intensive Vascular Care (PreDIVA)24 trials—yielded inconsistent results. FINGER demonstrated some benefit, but MAPT and PreDIVA did not, though post-hoc analyses of participants at high dementia risk suggested benefits. Overall, our findings along with previous studies suggest that promoting a healthy lifestyle could be a feasible strategy that could have an impact on prevention for cognitive impairment and dementia. The associations between healthy lifestyles and cognitive function are biologically possible. Physical activity is associated with increased brain volume, elevated brain-derived neurotrophic factor levels, reduced psychological stress, reduced cardiometabolic risk factor levels, and enhanced amyloid beta clearance25. Smoking exerts toxic effects through oxidative stress, neuroinflammation, and increase cardiometabolic risks25. Healthy diets rich in nutrients and vitamins may ameliorate cognitive impairment through their effects on oxidative stress, inflammation, and cardiometabolic health25.
We found a larger effect of SES on cognitive function. Considerable evidence shows that SES components are associated with cognitive function. Higher education levels are consistently associated with higher cognitive function and lower risk of dementia5,6. Lower income and sustained financial hardship were associated with lower cognitive function26,27, and food insecurity adversely impacts the cognitive function28. Studies examining the relationship between longest-held occupations and cognition found that blue collar occupations were associated with higher risks of cognitive impairment and Alzheimer’s disease29,30. A large cohort study of U.S. found that low access to health care was associated with 25% higher odds of cognitive impairment31. Our analysis observed similar associations between these single socioeconomic variables and cognitive function. More importantly, we constructed a composite SES variable given the nature of SES as a multidimensional construct comprising diverse socioeconomic factors, and that SES may affect health through multiple pathways32. The association between composite SES and cognitive function suggests the socioeconomic inequalities in cognitive health in U.S. older adults, and thus, exploring strategies to reduce socioeconomic inequalities is needed.
The contribution of healthy lifestyles to the socioeconomic inequalities in health is widely discussed, particularly on cardiovascular outcomes and mortality. A recent systematic review estimated that lifestyles factors were responsible for approximately 20% of the socioeconomic inequalities in health, suggesting the promotion of healthy lifestyles since they may help alleviate socioeconomic inequalities in health15. Contrary to these findings, we found that the healthy lifestyle score and SES were independently associated with cognitive function, though a borderline interaction between the healthy lifestyle score and SES was observed. Effect estimates of SES on cognitive function were also larger than those of the healthy lifestyle score. These findings may suggest that significant reductions in socioeconomic inequalities in health could not be achieved by only promoting healthy lifestyles, and additional modifiable factors such as environmental exposures, psychosocial factors, structural factors, and policies should be considered and evaluated. Some data suggests that SES inequality per se is a significant driver of health disparities. On the other hand, Lynch argues persuasively that more systemic interventions are required to address SES disparities in population health33. Addressing systemic factors, such as access to affordable and nutritious food, safe recreational spaces, and comprehensive healthcare services, can create an environment that supports the adoption and maintenance of healthy behaviors34. Further research, particularly prospective cohort studies or analyses of existing lifestyle intervention trial datasets, is needed to confirm our findings and provide a more comprehensive understanding of the complex interplay between healthy lifestyles, SES, and specific health outcomes.
Strengths of the current study include examining the associations of healthy lifestyles and SES with cognitive function in a representative sample of U.S. older adults. In addition, we constructed a healthy lifestyle score and composite SES, acknowledging the complex and multifactorial impacts of lifestyle and socioeconomic factors on cognitive function. However, we acknowledge that the cross-sectional nature of NHANES data precludes the ability to assess longitudinal cognitive decline. We could not rule out the possibility of reverse causality that participants’ cognitive function could impact self-report on behavior factors such as diet. Additionally, both lifestyle and socioeconomic factors were measured in late life; thus, we are not able to investigate whether lifestyle and SES changes across the life course are associated with cognitive function at older ages. Therefore, it is critically important to examine the contribution of SES and lifestyle to cognition using the life course approach. Future studies with longitudinal design will also enable us to better understand the causal relationships between SES, healthy lifestyle, and health outcomes by employing causal mediation analysis. Finally, we were unable to completely eliminate residual confounding due to limited categorization of covariates, for example, lack of non-binary gender identity35 and APOE genotype36 due to the limitation in NHANES measures, despite we have controlled for many known confounders.
Our study provides evidence that higher SES, and healthy lifestyle are independently associated with a higher cognitive function performance in U.S. older adults. The magnitude of the SES association with cognitive function was considerably larger than the association between healthy lifestyle factors and cognitive performance. No statistically significant interaction between the healthy lifestyle and SES was observed, suggesting that reductions in socioeconomic inequalities in health cannot not be achieved by only promoting healthy lifestyles. Future studies with longitudinal design are needed to confirm our findings and explore more modifiable factors that help mitigate socioeconomic inequalities in health.
Methods
Study population
Data were from the NHANES, a large, nationally representative cross-sectional survey and physical examination conducted in 2-year cycles, assessing the health and nutritional status of the civilian, noninstitutionalized United States population. Details of the survey and laboratory procedures are published elsewhere37. The comprehensive cognitive evaluation was available among 2937 adults ≥ 60 years old from two continuous NHANES data releases: 1364 from the 2011–2012 cycle and 1573 from the 2013–2014 cycle. Our samples excluded 1624 participants without information on lifestyle factors, SES factors, and covariates. Our final analytic sample included 1313 participants from the NHANES 2011–2014 (Figure S1).
Standard protocol approvals, registrations, and patient consents
The NHANES protocol followed the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the National Center for Health Statistics Research Ethics Review Board, and written informed consent was obtained from all participants or legally authorized representatives. The National Center for Health Statistics Research Ethnics Review Board approved all study protocols, and written informed consent was obtained from all participants. All methods were performed in accordance with relevant guidelines and regulations and followed the Strengthening the Reporting of Observational Studies for Epidemiology (STROBE) guidelines38.
Lifestyle factor assessment
We calculated a composite healthy lifestyle score consisting of multiple domains, including self-reported cigarette smoking, alcohol use, physical activity, and diet, which align with previous NHANES studies and recommendations set forth by the World Health Organization39,40,41. Information on all lifestyle factors were collected using structured questionnaires or 24-h dietary recall. Cigarette smoking was categorized into never smoking (smoked fewer than 100 cigarettes in lifetime) or smoking history, with never smoking considered a healthy level. Alcohol use was defined by daily consumption of alcohol, and a healthy level was defined as daily consumption of no more than one drink for women and no more than two drinks for men42. Physical activity was assessed weekly by metabolic equivalent hours of leisure time (MET). MET was categorized into tertiles, and we considered participants in the top tertile to show evidence of adequate physical activity (healthy level).
Diet was characterized by the healthy eating index-2015 (HEI-2015)43. The HEI-2015 is a diet quality index to assess the adherence to 2015–2020 Dietary Guidelines for Americans43. Intake of each HEI-2015 component was scored proportionately between the minimum and maximum standards. The details of HEI-2015 components and scoring standards are shown in Table S1. HEI-2015 scores range from 0 to 100. A healthy diet was defined as HEI-2015 scores in the top 40%, while those in the bottom 60% showed evidence of unhealthy eating39,40.
We constructed a composite healthy lifestyle score. For each lifestyle factor, we assigned 1 point for a healthy level and 0 point for an unhealthy level. The healthy lifestyle score was calculated by summing each individual lifestyle factor score, and possible scores ranged from 0 to 4. Higher healthy lifestyle scores indicated greater adherence to healthy behaviors. Healthy lifestyle scores were classified into approximate tertiles, comprising 0–1, 2, and 3–4 healthy behaviors.
SES assessment
The present study focused on individual-level SES, characterized by education, family income, occupation, health insurance, and food security44. Education levels were categorized into less than high school, high school or equivalent, and college or above. Family income levels were defined as the ratios of family income to the poverty thresholds specific to the survey year. Family income levels were classified into low income (family income to poverty ratio ≤ 1), middle income (> 1 and < 4), and high income (≥ 4)45. Occupation was defined by each participant’s longest job, and categorized as blue collar (service, farming/forestry/fishing, precision production/craft/repair, and operator/fabricator/laborer), white collar (managerial/professional specialty, and technical/sales/administrative support), unemployed, or not in labor force (retired persons, homemakers, and students)46. Health insurance was classified into government insurance (Medicare, Medi-Gap, Medicaid, State Children’s Health Insurance Program, military health plan, Indian Health Service, state-sponsored health plan, or other government insurance), private insurance, both government and private insurance, or uninsured. Food insecurity was measured using 18-item Food Security Survey Module, and dichotomized into full food security and food insecurity47.
For composite SES, LCA was used to identify the presence of underlying constructs (or classes) in the observed SES components (education, occupation, income-to-poverty ratio, health insurance, and food security)48. LCA estimates conditional class membership probability and classifies individuals who are homogeneous in terms of particular criteria. We compared the performance of LCA with two, three, and four classes, and the optimal number of latent classes was determined based on the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC)48. We identified three latent classes—high, medium, and low SES groups.
Cognitive function assessment
Comprehensive cognitive testing was completed by participants aged ≥ 60 years during NHANES 2011–2012 and 2013–2014 cycles. Four tests were administered, including the CERAD Word Learning subtests to evaluate immediate and delayed learning ability49, the Animal Fluency test to assess categorical verbal fluency (component of executive function)50, and the DSST from the Wechsler Adult Intelligence Scale to assess processing speed, sustained attention, and working memory51. Individual results of the four tests (CERAD immediate and delayed learning, Animal Fluency, DSST) converted to z-scores using age appropriate normal means. Individual test z scores are averaged to form the cognitive composite z-score, similar to previous studies52.
Covariates
Covariates were selected based on previous research and included age, sex, race/ethnicity, marital status, birthplace, and BMI53. Sex included male and female. Participants were assigned to one of these categories by NHANES based on their questionnaire responses. Race/ethnicity categories provided by NHANES included non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, Hispanic, or other races including multiracial. Marital status included never married, married, or not married (including widowed, divorced, separated, or living with partner). Birthplace was categorized as born in or outside of the United States. BMI (kg/ m2) was computed as the ratio of weight (kg) and height squared (m2).
Statistical analyses
Mean and standard error (SE) were computed for continuous variables, and percentage (%) was calculated for categorical variables. Participant characteristics, composite cognition z-scores, and scores of the four individual cognitive tests were compared by composite healthy lifestyle and composite SES groups. The sampling weights and design variables were used for all analyses.
In the primary analysis, we utilized survey-weighted linear regression models to examine the associations of healthy lifestyle score and composite SES derived from LCA with composite cognition z-score, after adjusting for age, sex, race/ethnicity, marital status, and birthplace. To assess the joint associations of lifestyles and SES, we classified participants into nine groups based on three SES classes (low, medium, high) and three healthy lifestyle groups (0–1, 2, 3–4). Differences in cognition z-score and 95% confidence intervals were calculated with participants with low SES and 0–1 health lifestyle as the reference group. Likelihood-ratio test comparing models with and without interaction terms between SES and healthy lifestyle groups was used to test the interaction between healthy lifestyle score and composite SES.
In the secondary analysis of individual SES and lifestyle factors, we performed linear regression models to estimate differences in composite cognitive z-score and 95% confidence intervals. Model 1 included age, sex, race/ethnicity, marital status, birthplace, and individual lifestyle factors (smoking status, alcohol consumption, physical activity, and HEI-2015). Model 2 included age, sex, race/ethnicity, marital status, birthplace, and individual SES factors (education, family income, occupation, health insurance, and food security). Model 3 included age, sex, race/ethnicity, marital status, birthplace, as well as both individual lifestyle and SES components.
To test the robustness of our findings, we conducted several sensitivity analyses. First, to assess the different cognitive domains, we determined the relationships between lifestyle and SES factors with z-scores of each of the four cognitive test scores. Second, to assess potential differential influences between healthy lifestyle factors on cognition, we computed a weighted healthy lifestyle score. The weighted healthy lifestyle score was constructed as the sum of lifestyle factor scores where weights are beta coefficients of each individual lifestyle factor derived from Model 1 in the analysis of individual lifestyle factors. We then used this weighted healthy lifestyle score in a multivariable regression analysis. Furthermore, BMI could be an intermediate factor linking exposures and cognition, so we did not adjust for BMI in our primary analyses. However, in sensitivity analyses, we additionally controlled for BMI. Moreover, we repeated our analyses in a subpopulation excluding participants with stroke or cardiovascular disease, which might impair cognitive functions54. We did not excluding these participants in the primary analysis in consideration of potential overadjustment bias because stroke and cardiovascular disease may serve as risk factors for cognitive decline and could be also influenced by SES and healthy lifestyle factors. Finally, to explore the impact of missing values on the observed results, we conducted multiple imputations with chain equation to impute missing values55. This procedure used PROC MI in SAS to create 20 datasets for missing values and computed pooled effect estimates using PROC MIANALYZE. We repeated Models 1–3 on each of the imputed datasets and compared the effect estimates to the primary findings using measured data. All statistical analyses were performed using SAS (version 9.4, SAS Institute Inc.). Statistical significance was set at a two-sided P < 0.05.
Data availability
All data and materials have been made publicly available at the National Center for Health Statistics website (https://www.cdc.gov/nchs/nhanes/index.htm).
Abbreviations
- AIC:
-
Akaike Information Criterion
- BIC:
-
Bayesian Information Criterion
- BMI:
-
Body mass index
- CERAD:
-
Consortium to Establish a Registry for Alzheimer’s Disease
- DSST:
-
Digit Symbol Substitution Test
- FINGER:
-
Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability
- HEI:
-
Healthy eating index
- LCA:
-
Latent class analysis
- MAPT:
-
French Multidomain Alzheimer Preventive Trial
- MET:
-
Metabolic equivalent hours of leisure time
- NHANES:
-
National Health and Nutrition Examination Survey
- PreDIVA:
-
Dutch Prevention of Dementia by Intensive Vascular Care
- SES:
-
Socioeconomic status
References
-
World Health Organization. The Global Dementia Observatory Reference Guide World Health Organization. (2018).
-
Cerhan, J. R. et al. Correlates of cognitive function in middle-aged adults. Gerontology 44, 95–105 (1998).
-
Freidl, W. et al. Mini mental state examination: Influence of sociodemographic, environmental and behavioral factors, and vascular risk factors. J. Clin. Epidemiol. 49, 73–78 (1996).
-
Koster, A. et al. Socioeconomic differences in cognitive decline and the role of biomedical factors. Ann. Epidemiol. 15, 564–571 (2005).
-
Letenneur, L. et al. Are sex and educational level independent predictors of dementia and Alzheimer’s disease? Incidence data from the PAQUID project. J. Neurol. Neurosurg. Psychiatry 66, 177–183 (1999).
-
Alley, D., Suthers, K. & Crimmins, E. Education and Cognitive Decline in Older Americans: Results From the AHEAD Sample. Res. Aging 29, 73–94 (2007).
-
Hackman, D. A., Farah, M. J. & Meaney, M. J. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nat. Rev. Neurosci. 11, 651–659 (2010).
-
Bennett, H. Q. et al. Healthy ageing for all? Comparisons of socioeconomic inequalities in health expectancies over two decades in the Cognitive Function and Ageing Studies I and II. Int. J. Epidemiol. 50, 841–851 (2021).
-
Cummings, J. et al. Drug development in Alzheimer’s disease: The path to 2025. Alzheimer’s Research and Therapy 8, (2016).
-
Norton, S., Matthews, F. E., Barnes, D. E., Yaffe, K. & Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. The Lancet Neurology 13, 788–794 (2014).
-
Ngandu, T. et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. The Lancet 385, 2255–2263 (2015).
-
Zhong, G., Wang, Y., Zhang, Y., Guo, J. J. & Zhao, Y. Smoking is associated with an increased risk of dementia: A meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS ONE 10, (2015).
-
Guure, C. B., Ibrahim, N. A., Adam, M. B. & Said, S. M. Impact of Physical Activity on Cognitive Decline, Dementia, and Its Subtypes: Meta-Analysis of Prospective Studies. BioMed Research International 2017, (2017).
-
Loef, M. & Walach, H. Fruit, vegetables and prevention of cognitive decline or dementia: A systematic review of cohort studies. Journal of Nutrition, Health and Aging 16, 626–630 (2012).
-
Petrovic, D. et al. The contribution of health behaviors to socioeconomic inequalities in health: A systematic review. Prev. Med. 113, 15–31 (2018).
-
Lövdén, M., Fratiglioni, L., Glymour, M. M., Lindenberger, U. & Tucker-Drob, E. M. Education and Cognitive Functioning Across the Life Span. Psychological Science in the Public Interest 21, 6–41 (2020).
-
Opdebeeck, C., Martyr, A. & Clare, L. Cognitive reserve and cognitive function in healthy older people: A meta-analysis. Aging Neuropsychol. Cogn. 23, 40–60 (2016).
-
Krieger, N., Williams, D. R. & Moss, N. E. Measuring social class in us public health research: Concepts, methodologies, and guidelines. Annu. Rev. Public Health 18, 341–378 (1997).
-
Liberatos, P., Link, B. G. & Kelsey, J. L. The measurement of social class in epidemiology. Epidemiol. Rev. 10, 87–121 (1988).
-
Lourida, I. et al. Mediterranean diet, cognitive function, and dementia: A systematic review. Epidemiology 24, 479–489 (2013).
-
Smith, P. J. et al. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom. Med. 72, 239–252 (2010).
-
Deckers, K. et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int. J. Geriatr. Psychiatry 30, 234–246 (2015).
-
Vellas, B. et al. MAPT Study: A Multidomain Approach for Preventing Alzheimer’s Disease: Design and Baseline Data. The Journal Of Prevention of Alzheimer’s Disease 1, 13–22 (2014).
-
van Charante, E. P. M. et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. The Lancet 388, 797–805 (2016).
-
Kivipelto, M., Mangialasche, F. & Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nature Reviews Neurology 2018 14:11 14, 653–666 (2018).
-
Lee, S., Buring, J. E., Cook, N. R. & Grodstein, F. The Relation of Education and Income to Cognitive Function among Professional Women. Neuroepidemiology 26, 93–101 (2006).
-
Zeki Al Hazzouri, A. et al. Sustained Economic Hardship and Cognitive Function: The Coronary Artery Risk Development in Young Adults Study. American Journal of Preventive Medicine 52, 1–9 (2017).
-
Na, M. et al. Food Insecurity and Cognitive Function in Middle to Older Adulthood: A Systematic Review. Adv. Nutr. 11, 667–676 (2020).
-
Helmer, C. et al. Occupation during life and risk of dementia in French elderly community residents. J. Neurol. Neurosurg. Psychiatry 71, 303–309 (2001).
-
Kim, H. J., Min, J. Y. & Min, K. B. The Association between Longest-Held Lifetime Occupation and Late-Life Cognitive Impairment: Korean Longitudinal Study of Aging (2006–2016). International Journal of Environmental Research and Public Health 2020, Vol. 17, Page 6270 17, 6270 (2020).
-
Mullins, M. A., Bynum, J. P. W., Judd, S. E. & Clarke, P. J. Access to primary care and cognitive impairment: results from a national community study of aging Americans. BMC Geriatr. 21, 1–10 (2021).
-
Braveman, P. A. et al. Socioeconomic Status in Health Research: One Size Does Not Fit All. JAMA 294, 2879–2888 (2005).
-
Lynch, J. Reframing inequality? The health inequalities turn as a dangerous frame shift. J. Public Health 39, 653–660 (2017).
-
Diez Roux, A. V. & Mair, C. Neighborhoods and health. Annals of the New York Academy of Sciences 1186, 125–145 (2010).
-
Rocca, W. A., Mielke, M. M., Vemuri, P. & Miller, V. M. Sex and gender differences in the causes of dementia: A narrative review. Maturitas 79, 196–201 (2014).
-
Key, F. P. et al. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature Reviews Neurology 2013 9:2 9, 106–118 (2013).
-
Centers for Disease Control and Prevention. NHANES – About the National Health and Nutrition Examination Survey.
-
Vandenbroucke, J. P. et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 12, 1500–1524 (2014).
-
Zhang, Y.-B. et al. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. BMJ 373, n604 (2021).
-
Ford, E. S., Zhao, G., Tsai, J. & Li, C. Low-Risk lifestyle behaviors and all-cause mortality: Findings from the national health and nutrition examination survey III mortality study. Am. J. Public Health 101, 1922–1929 (2011).
-
World Health Organization. Tackling NCDs: ‘best buys’ and other recommended interventions for the prevention and control of noncommunicable diseases. https://apps.who.int/iris/handle/10665/259232 (2017).
-
USDA. Dietary guidelines for Americans 2020–2025: Make every bite count with de Dietary Guidelines. 150 (2020).
-
Krebs-Smith, S. M. et al. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 118, 1591–1602 (2018).
-
Diemer, M. A., Mistry, R. S., Wadsworth, M. E., López, I. & Reimers, F. Best practices in conceptualizing and measuring social class in psychological research. Anal. Soc. Issues Public Policy 13, 77–113 (2013).
-
Odutayo, A. et al. Income disparities in absolute cardiovascular risk and cardiovascular risk factors in the United States, 1999–2014. JAMA Cardiology 2, 782–790 (2017).
-
Crespo, C. J., Smit, E., Andersen, R. E., Carter-Pokras, O. & Ainsworth, B. E. Race/ethnicity, social class and their relation to physical inactivity during leisure time: Results from the Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Prev. Med. 18, 46–53 (2000).
-
USDA. Household Food Security in the United States in 1995: Summary Report of the Food Security Measurement Project | Food and Nutrition Service. (1995).
-
Andersen, R., Hagenaars, J. A. & McCutcheon, A. L. Applied Latent Class Analysis. Canadian Journal of Sociology / Cahiers canadiens de sociologie vol. 28 (2003).
-
Morris, J. C. et al. The consortium to establish a registry for alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of alzheimer’s disease. Neurology 39, 1159–1165 (1989).
-
Selnes, O. A. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Neurology 41, 1856–1856 (1991).
-
Wechsler, D. WAIS-III: Wechsler Adult Intelligence Scale 3rd edn. (The Psychological Corporation, 1997).
-
Donohue, M. C. et al. The Preclinical Alzheimer Cognitive Composite: Measuring Amyloid-Related Decline. JAMA Neurol. 71, 961–970 (2014).
-
Park, S. K., Ding, N. & Han, D. Perfluoroalkyl substances and cognitive function in older adults: Should we consider non-monotonic dose-responses and chronic kidney disease?. Environ. Res. 192, 110346 (2021).
-
Gorelick, P. B. et al. Vascular Contributions to Cognitive Impairment and Dementia. Stroke 42, 2672–2713 (2011).
-
van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Acknowledgements
The authors would also like to thank the NHANES participants and the staff members for their contribution to data collection and for making the data publicly available.
Funding
This study was supported by grants from the National Institute on Aging (NIA) R01-AG070897, the National Institute of Environmental Health Sciences (NIEHS) P30-ES017885, the Center for Disease Control and Prevention (CDC)/National Institute for Occupational Safety and Health (NIOSH) T42-OH008455, and NIH/NIA Michigan Alzheimer’s Disease Research Center grant P30-AG072931 and the University of Michigan Alzheimer’s Disease Center (Berger Endowment).
Author information
Authors and Affiliations
Contributions
X.W. is the guarantor of this work and had full access to all the data in the study and takes responsibility for the contents of the manuscript. X.W. designed the study, conducted data analysis, and wrote the manuscript. K.M.B., H.L.P., R.L.A., and S.K.P. were involved in the design of the analysis plan, contributed to interpretation of the data, and critically revised the manuscript. All authors read and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Bakulski, K.M., Paulson, H.L. et al. Associations of healthy lifestyle and socioeconomic status with cognitive function in U.S. older adults. Sci Rep 13, 7513 (2023). https://doi.org/10.1038/s41598-023-34648-0
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41598-023-34648-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.